Lung Ultrasound
How can lung US help me?
By Justin Bowra
Lung US is a comparatively recent addition to emergency US. Its systematic use was first described by Lichtenstein in his textbook ‘General ultrasound in the critically ill’ (Springer, 2002). Since then, critical care doctors and respiratory physicians worldwide have taken it up for a number of indications including:
Diagnosis of:
- Pleural fluid & pleural disease
- Pneumothorax (PTX)
- Pulmonary oedema
- Pneumonia
- Abscess
- Pulmonary contusion
- Pulmonary infarction
Procedural guidance for:
- Thoracocentesis
- Intercostal catheter placement
More generally in the critically ill patient:
- Cardiac arrest
- Respiratory distress
- Shock and fluid status
Why use ultrasound?
- Pneumothorax and massive haemothorax can be rapidly fatal if not detected and treated urgently
- Plain chest radiograph (CXR) can be unreliable
- Even with experience, differentiating between conditions such as pneumonia and pulmonary oedema can be challenging
- Lung US is easy to learn, non-invasive, rapid, repeatable and can be performed at the bedside
- Lung US is more sensitive and reliable than mobile CXR in the detection of pleural fluid: it can detect as little as 100ml, with sensitivity >97%, and specificity 99-100%.In expert hands, lung US has been described as 98% sensitive and 99% specific for PTX, and 85.7% sensitive and 98% specific for pulmonary oedema
- BUT lung US is very operator dependent! This means that it is less accurate in the hands of novice scanners
- Lung US improves the safety of invasive procedures
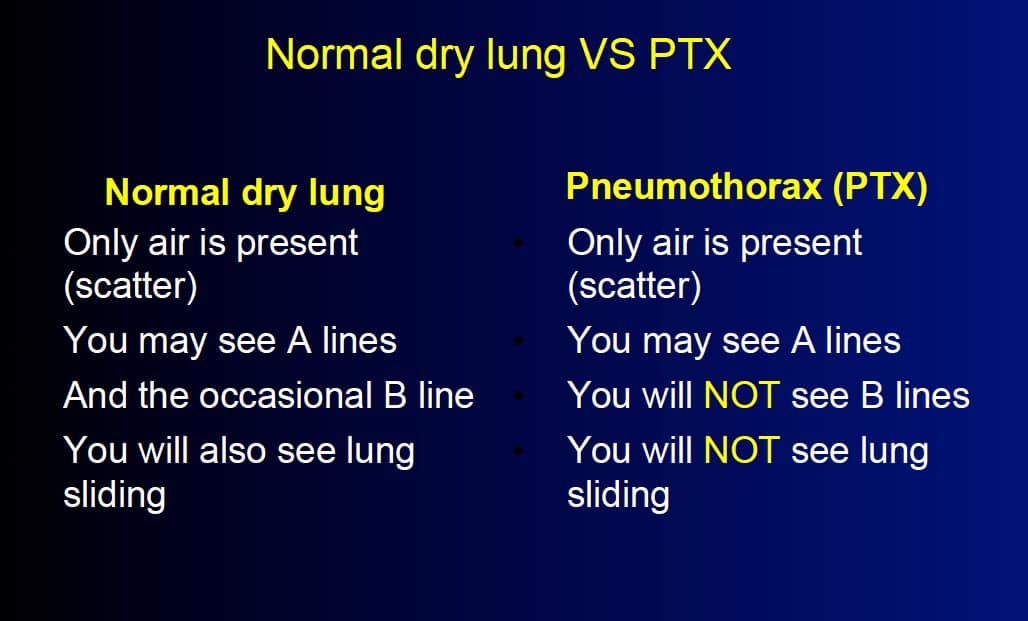
The US appearance of normal lung differs from disease processes that reach the pleural surface
Normal lung
- Air in lung reflects the sound
- You don’t really seeing normal lung at all
- Sparkle = scatter from air in lung
- Pleural line is a ‘twinkling curtain’ sliding back & forth
- Dry air: bright horizontal lines
Pathology
- PTX: air but no sliding
- Pleural fluid: ‘gap’ between chest wall & lung
- Pleural disease: thickened / irregular
- Wet / fibrosed lung: bright vertical lines
- Consolidated / infarcted lung: hypoechoic ‘chunks’
Probe and scanner settings
Probe
Are you in a hurry? Or performing a screening scan such as EFAST/CCUS?
The curved probe and sector (cardiac) probes are probably as good as each other. Every clinician sonologist has his/her preferences
(mine is the curved probe on the abdominal preset) but there’s no strong evidence either way.
It’s worth noting that the father of lung US, Daniel Lichtenstein, advises that a microconvex probe is best so try it if you have one. (I don’t.)
DON’T use the linear array probe if you can help it. It won’t show anatomical relations (eg you might place the probe on the liver and think it’s consolidated lung).
Are you looking for artifacts (sliding and B-lines)? Once again, avoid the linear probe. Its ability to image structures in fine detail means it tends to obliterate artifacts! This is particularly true for B lines.
By contrast, the very curvature of the images when the curved /sector probes are used tend to bring out the B lines, making them more obvious.
Are you looking for very fine detail e.g. pleural thickening, small areas of consolidation or tiny pneumothorax? Finally a role for the linear array probe! It is uniquely suited to imaging very superficial structures (in this case the pleural surface and sub-pleural pathology). But the curved probe is not bad here either.
Avoid the sector probe for fine imaging at the lung surface. Although it fits between ribs & gets round the back, its poor spatial resolution and poor near field detail make it the worst choice here.
Pre-set
Some machines come equipped with lung presets, although you can ‘do it yourself’ by:
- Selecting abdo / FAST preset
- Turning off the fancy filters e.g.
- THI (tissue harmonic imaging)
- Compounding/ multibeam (MB):
This is because the ‘fancy filters’ make the artifact (especially B lines) harder to see.
Depth
This depends on what you’re looking for.
- Just lung sliding, or fine detail at the pleural surface (eg microconsolidations): centre the pleural line on screen, which usually means 5cm depth.
- Differentiating dry, wet or chunky lung: 10 cm will do.
- Base of lungs e.g. mapping out an effusion: 15 cm sometimes needed.
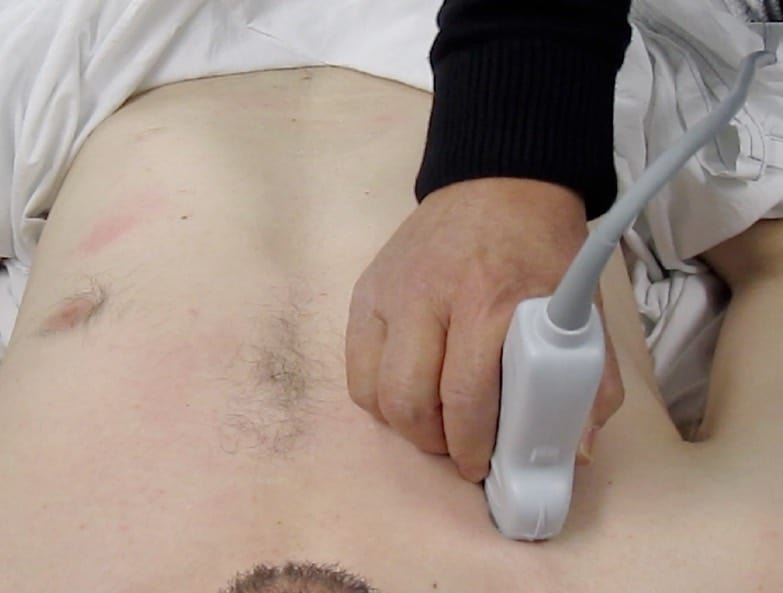
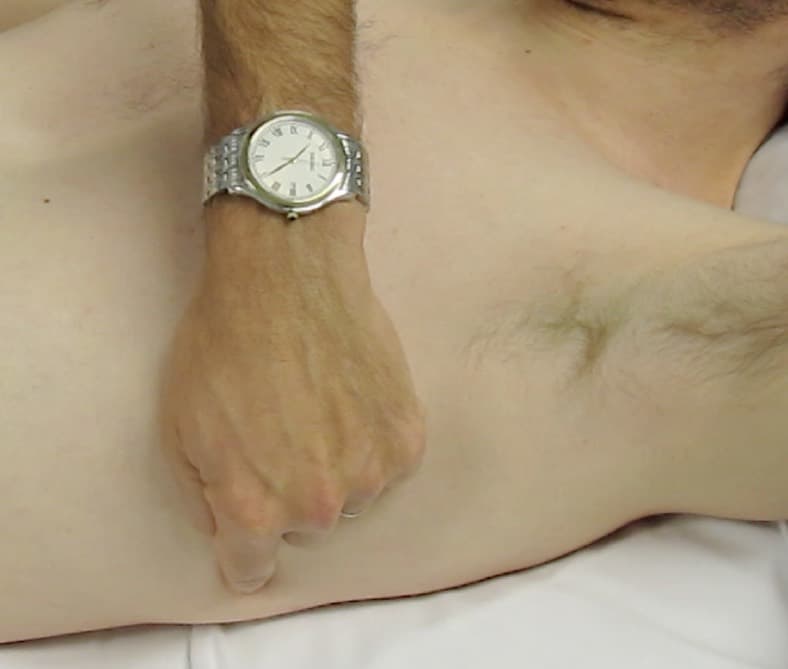
Holding the probe
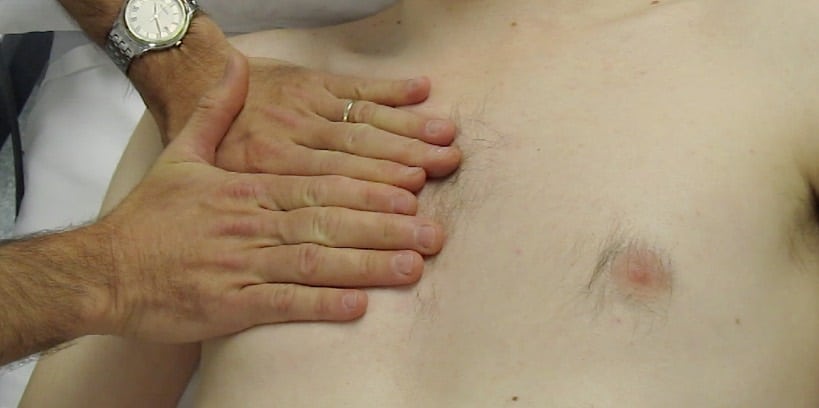
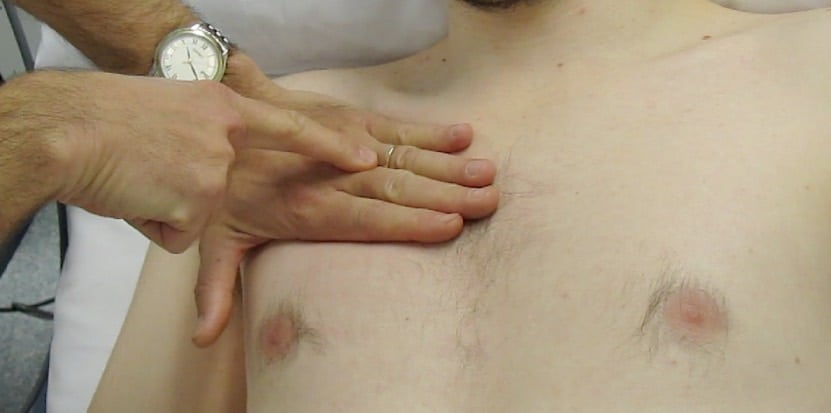
Hold the probe so that your hand rests comfortably on the patient’s skin, like holding a pen. (Figures 1 & 2 below)
This is for a number of reasons:
- Ergonomic: this is the most comfortable way to hold a probe, allowing the hand’s intrinsic muscles to delicately manipulate the probe while the forearm muscles can rest.
- Minimise probe movement: a key feature of LUS is to recognise the presence or absence of pleural movement (see below). If your hand is resting firmly on the patient’s skin, this will ensure that you minimise inadvertent probe movement (which can mimic the appearance of lung sliding, particularly on M-mode).
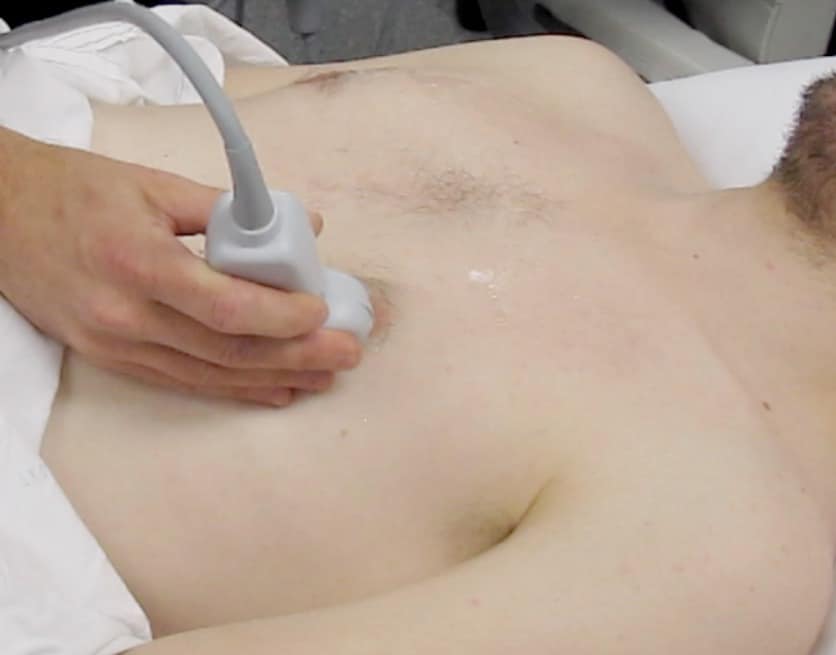
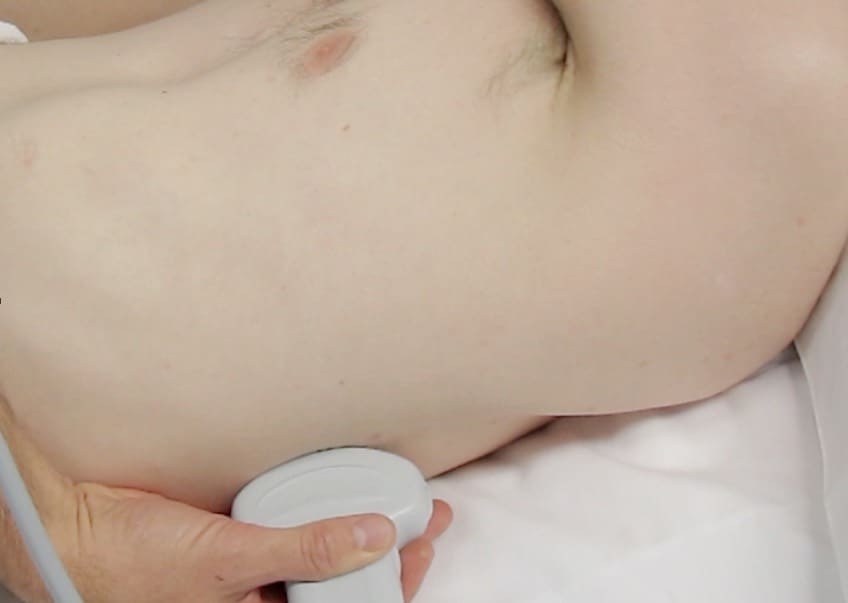
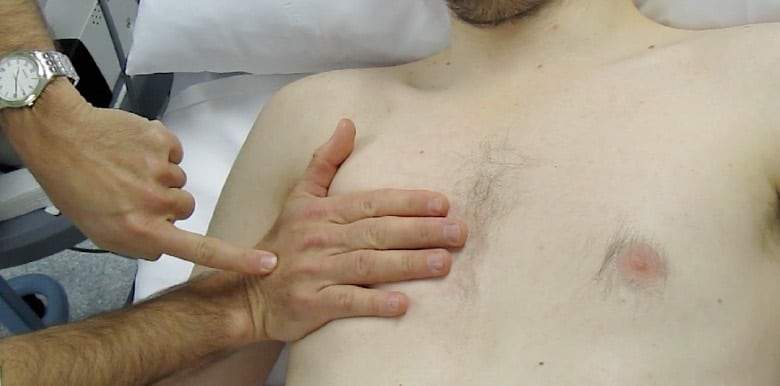
Placing the probe on the patient
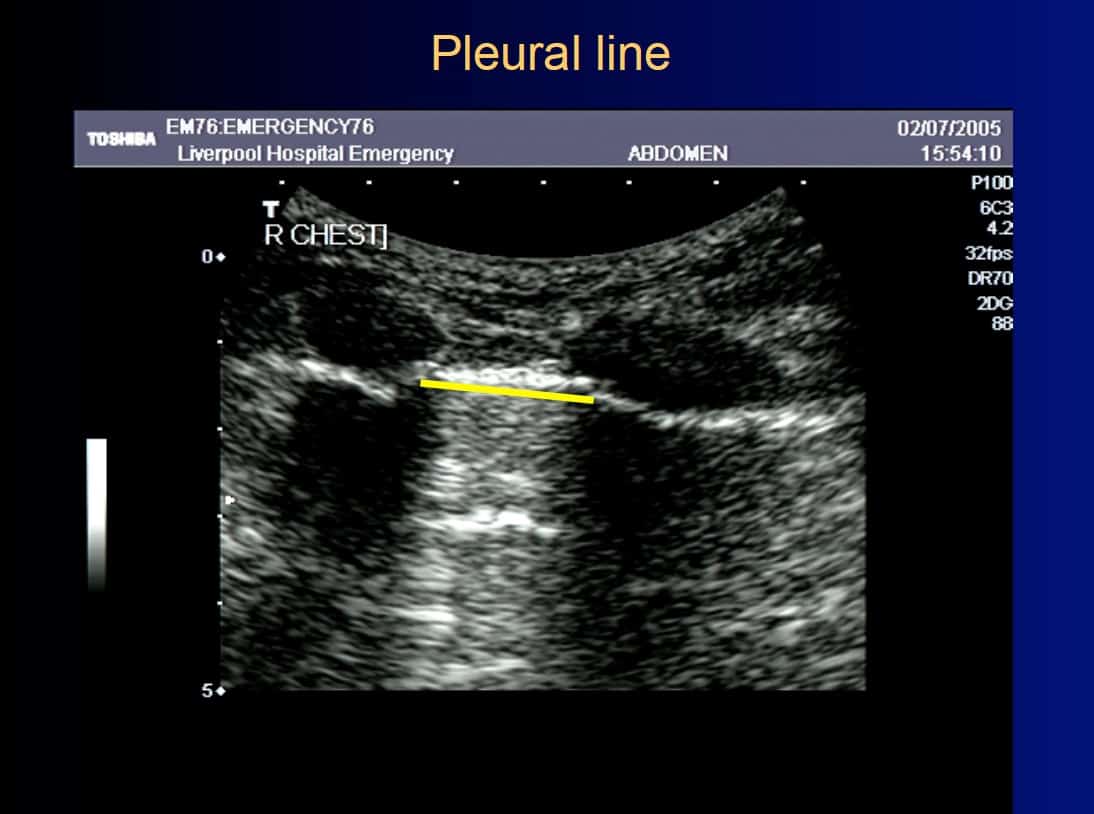
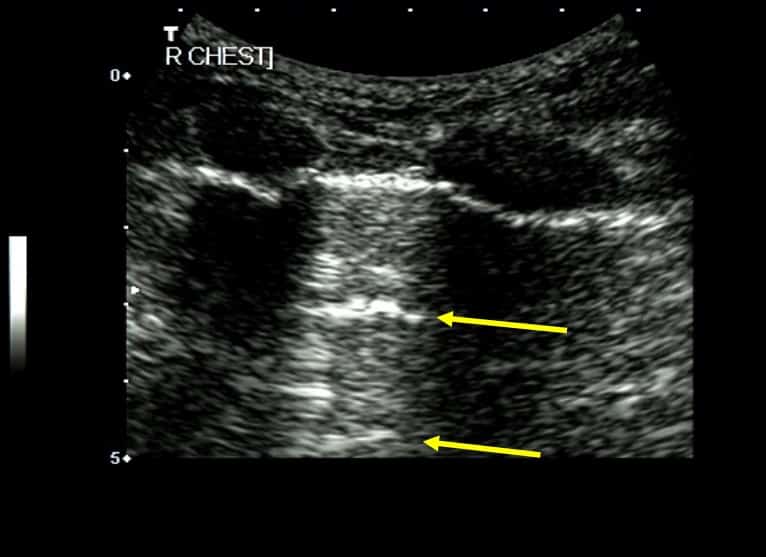
Ensure that you keep the probe in the long axis of the patient, rather than scanning between the ribs. (Figures above). This is because it is essential to visualise the posterior acoustic shadows cast by the ribs and costal cartilages, and the echogenic (bright) pleural line between and just below them. (highlighted in yellow, figure 3).
Once you have identified the pleural line, it is reasonable to scan parallel to the ribs to visualise as much of the lung surface as possible. However, it is not essential and carries the risk of inadvertently scanning the rib and mistaking this for lung pathology.
Where to scan?
This has been the subject of some controversy. However, the following is a practical guide:
- If scanning for pneumothorax, scan the highest part of the lung at the time (air rises). In a supine patient, this usually means the anterior chest. (Figure 1) If the patient is sitting up, a small pneumothorax may be missed because the apices are difficult to scan with US. Therefore lie the patient down if possible.
- If scanning for pleural fluid or haemothorax, scan the lowest part of the lung at the time (fluid sinks). In a supine patient, this usually means the posterior chest and more specifically the posterior costophrenic angle (Lichtenstein’s ‘PLAPS’ point, see appendix). (Figure 2)
- If scanning focal pathology (e.g. pleuritic pain), ask the patient to point to the site of maximum pain and scan there. (Be gentle if rib fracture is on the differential diagnosis!)
- If scanning for more diffuse disease such as pulmonary oedema, one needs to balance sensitivity with practicality. The more regions scanned, the more accurate the test will be but this can become time consuming.
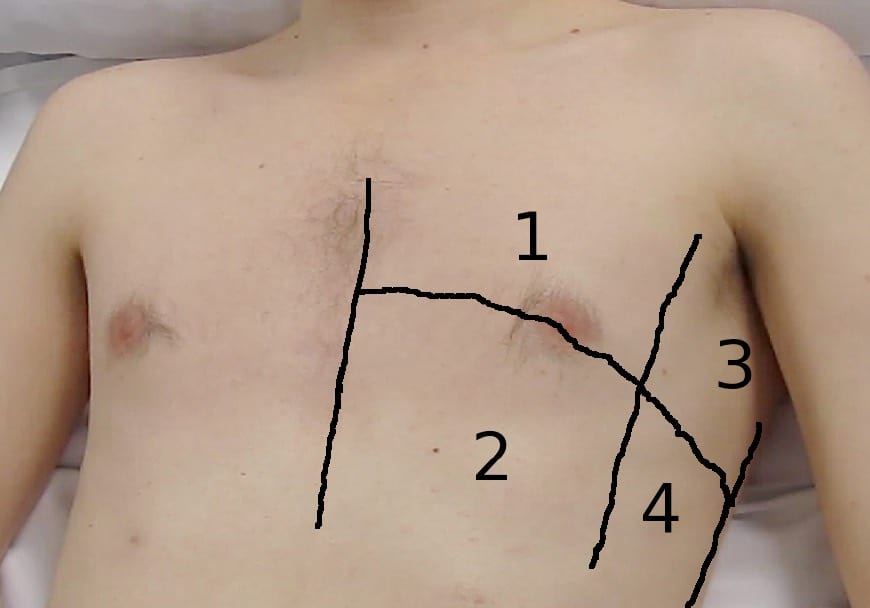
- The current consensus is to scan eight regions of the chest (four on each side) (Figure 8) but two other methods have been described:
- An anterior two-region scan is more rapid and may suffice to rule in/ out severe interstitial syndrome in the critically ill, but is probably insufficient to rule in/ out subtle pathology such as early pneumonia or mild heart failure
- A through evaluation of all 28 rib interspaces is sometimes advocated; this is best left to a thorough lung scan after initial resuscitation, as it is pretty time consuming!
Volpicelli’s eight-zone lung evaluation (Figure 4)
Each side of the chest can be demarcated by three vertical lines (parasternal, anterior axillary and posterior axillary) and one horizontal line into four areas:
- Area 1 = upper anterior
- Area 2 = lower anterior
- Area 3 = upper posterior
- Area 4 = lower posterior
Lichtenstein’s BLUE and PLAPS points
An alternative approach is outlined by Lichtenstein: the anterior chest is scanned at the upper and lower ‘BLUE’ points (roughly equivalent with the upper lobe and middle lobe/lingual respectively, and the posterior chest at the ‘PLAPS’ (posterolateral alveolar pleural syndrome) point: roughly equivalent with the lower lobe. (1) Both approaches appear equivalent, and the reader is encouraged to use whichever approach s/he finds more useful.
Want to know more about Lichtenstein’s method? See Appendix A the end of this manual.
What am I looking for? Image interpretation
To begin: five simple questions
1. Is there lung sliding?
2. Is there pleural fluid?
3. Are there A-lines, B-lines or no lines at all?
4. Is there consolidation?
5. Is the pleural line irregaular?
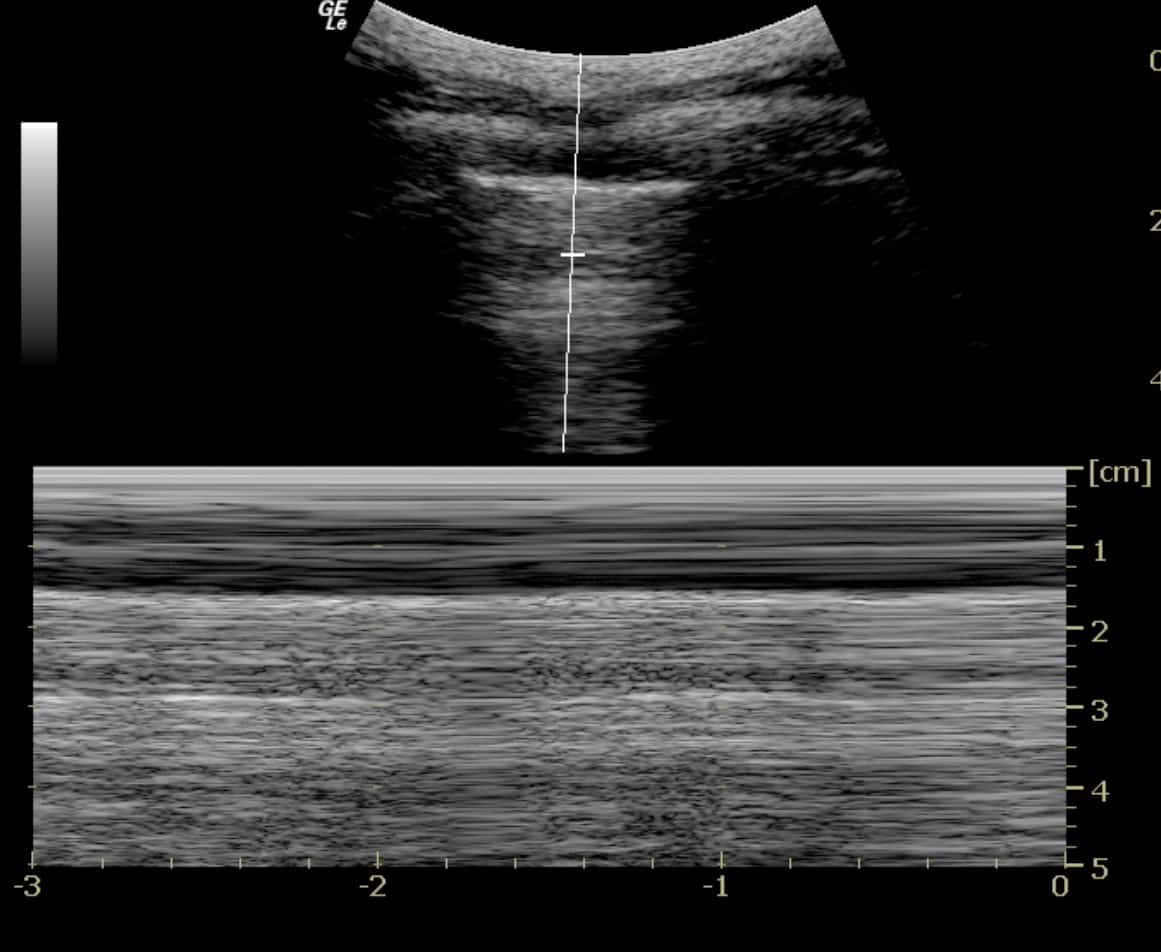
1. Is there lung sliding?
- The simplest LUS sign to describe
- But can be difficult to interpret in practice
- Lung sliding = the respiratory movement of the visceral on the parietal pleura
- Resembles ‘sparkling’ / ‘twinkling’ seen at the pleural line
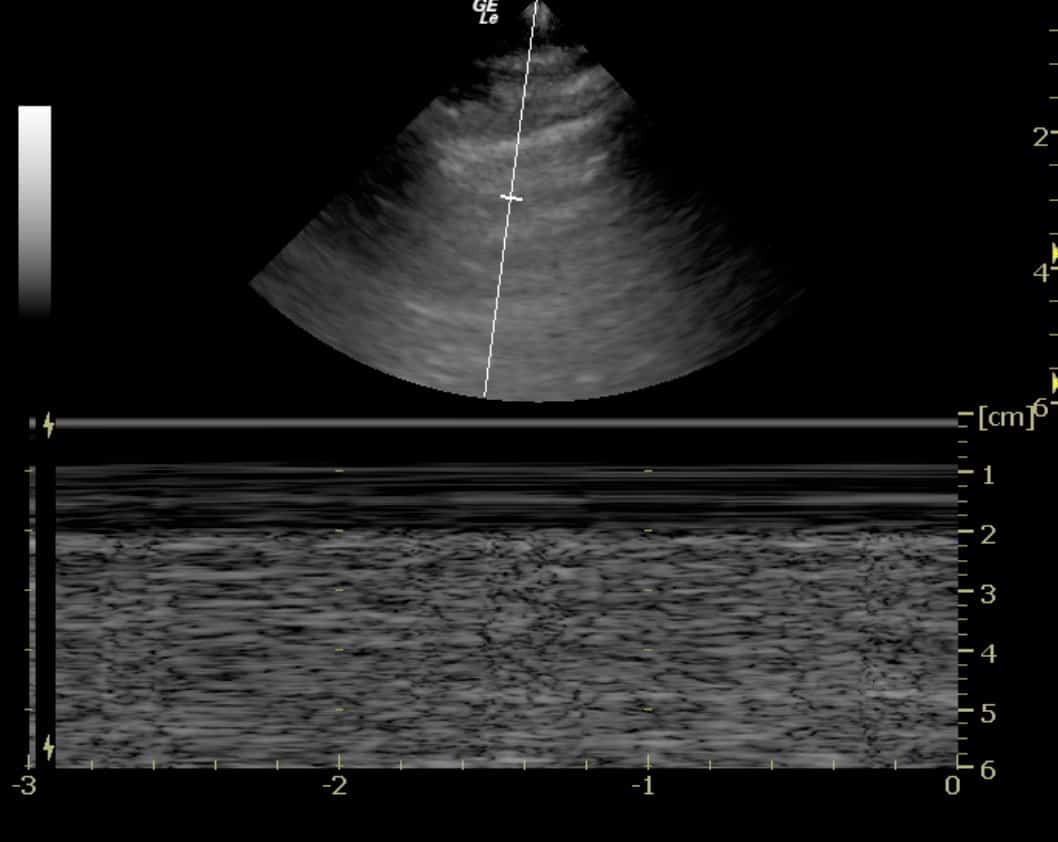
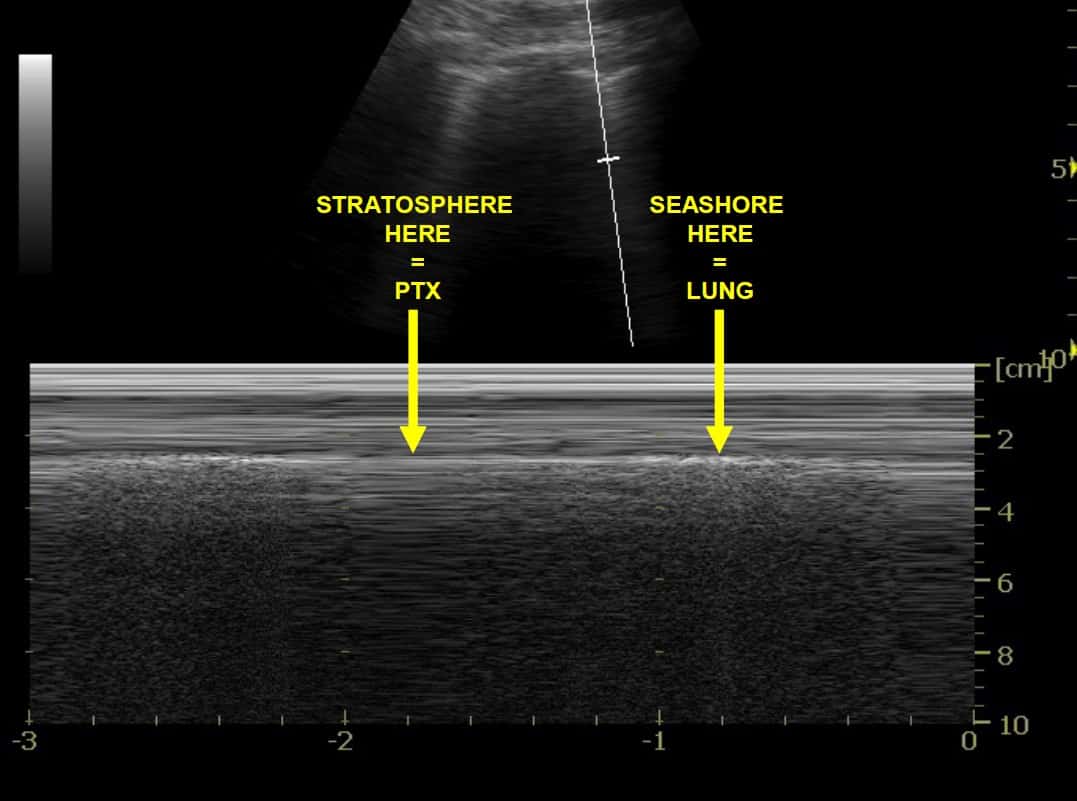
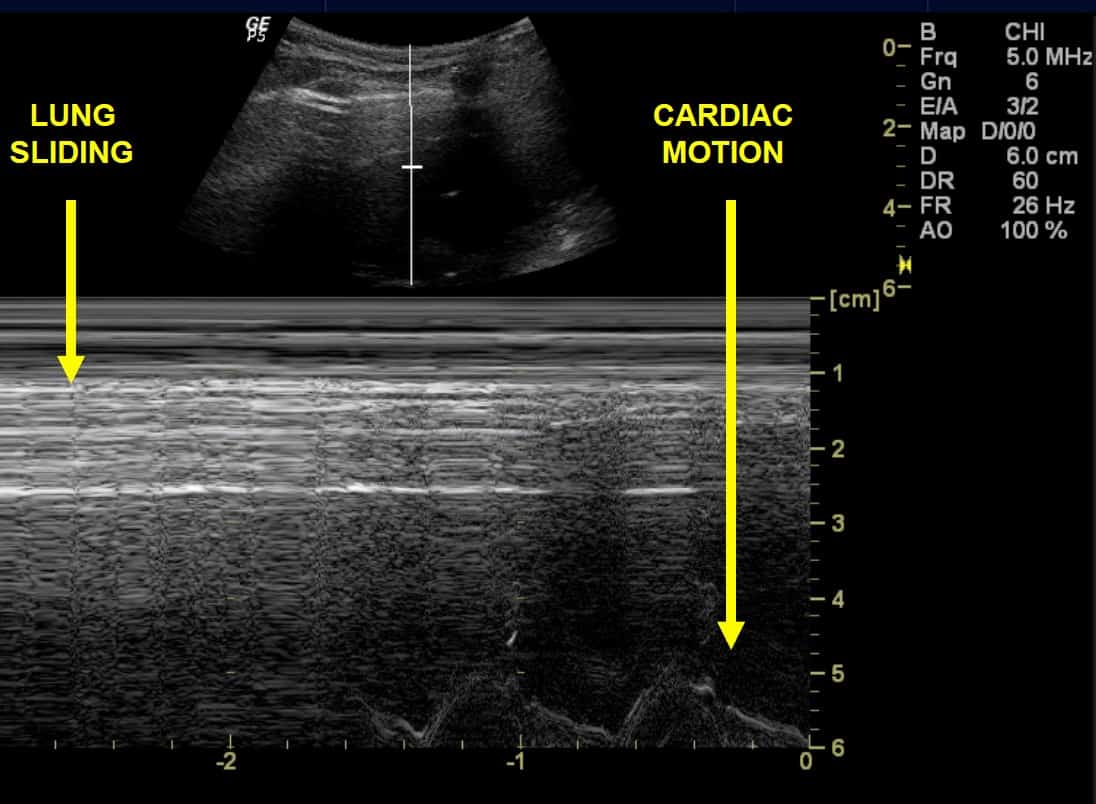
- If not obvious, M-mode (Motion mode) can be used:
a. Obtain a standard greyscale (B-mode) image of the pleural line as described above
b. Activate ‘M mode’ on your machine. On the screen, a vertical line will appear. Using the track ball/ track pad, move the line so that it intersects the pleural line rather than the ribs.
c. M-mode is ‘Motion mode’: in other words, this creates an image of what the vertical line ‘sees’ over time
- y-axis on an M-mode image = time
- x-axis = the vertical line. (Figure 5)
d. If a structure is not moving, it creates a series of horizontal lines (note the horizontal lines created by the
muscle, fascia, fat and skin above the pleural line seen in figure 5.
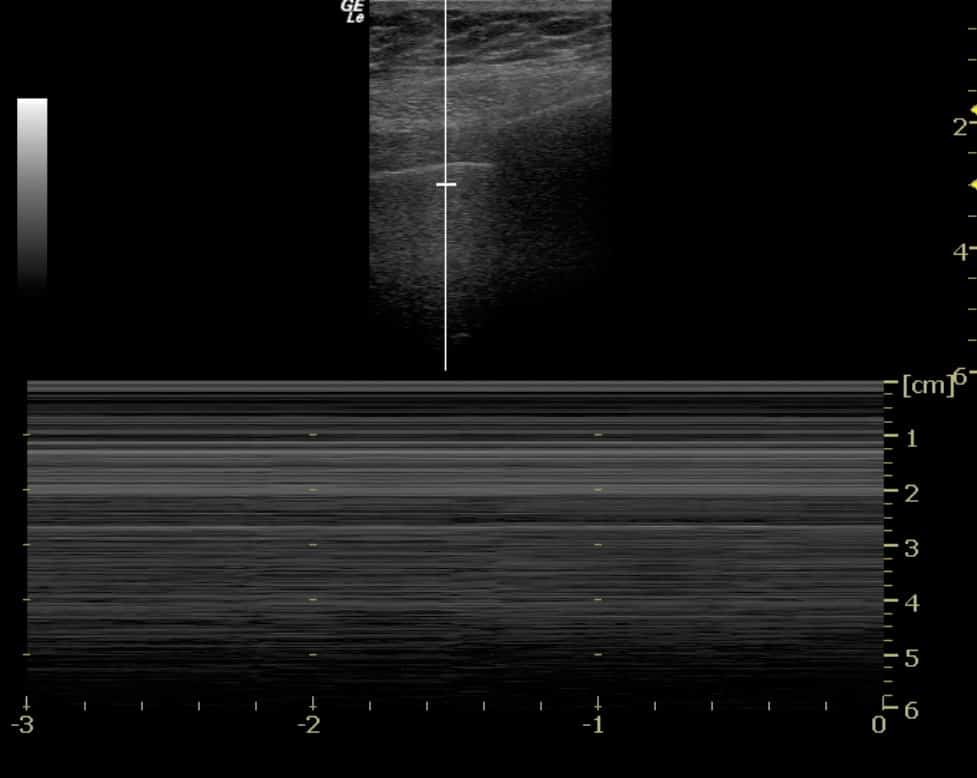
e. If a structure is sliding back and forth (such as the visceral pleura sliding on the parietal pleura in this context), then a ‘grainy’ image results, as in the grainy artifact seen below the pleural line in figure 6.
If no movement is seen at all below the pleura, then no lung sliding is occurring (Figure 6).
Why is lung sliding absent?
Lung sliding will be absent in several conditions:
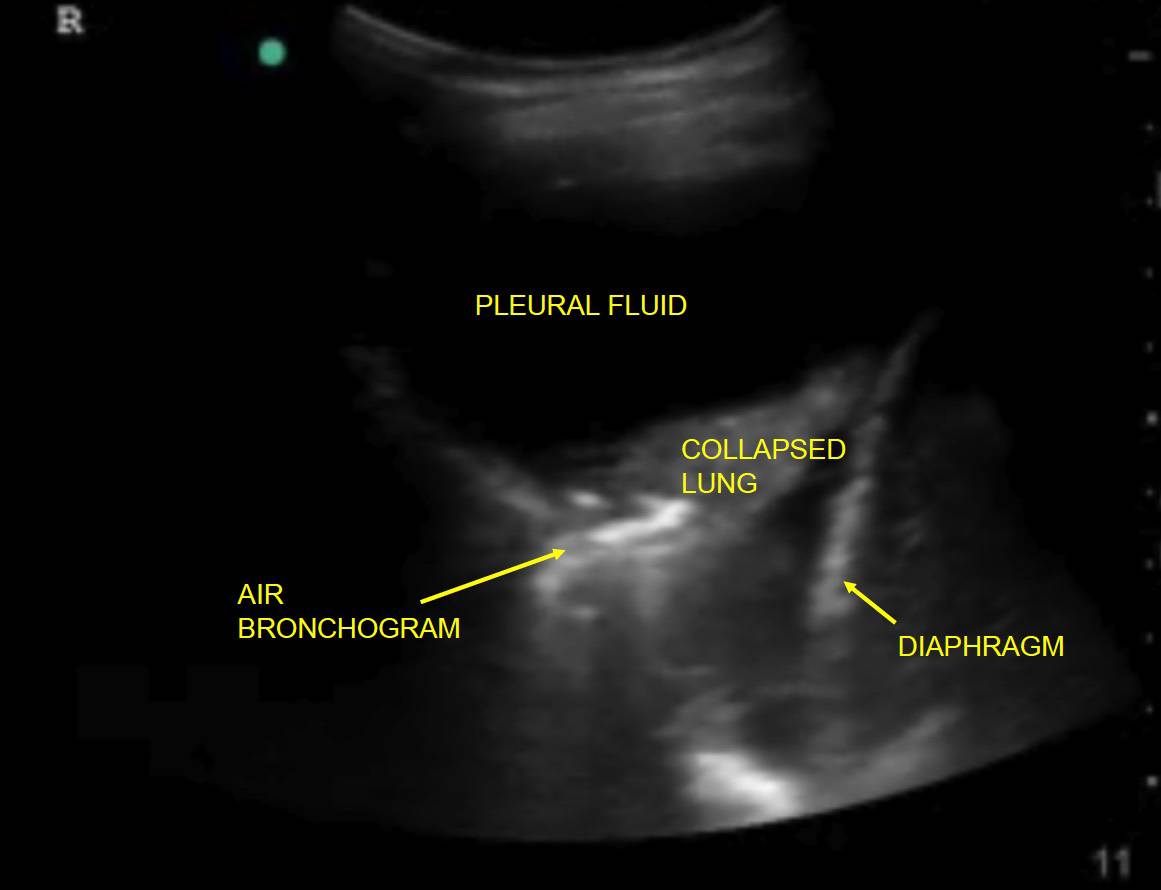
a. Pneumothorax (PTX) (figure 6) = the classic cause of absent lung sliding.
- The air in the PTX separates the two pleural layers i.e. the sound waves are ‘bouncing back’ to the probe from air within the thorax rather than in the lung
- The lung is trapped below the air in the pneumothorax
- So you can’t see the visceral pleura
- No contact between pleural layers = no lung sliding is seen on screen.
b. Lung sliding is often reduced or even absent in pneumonia and adult respiratory distress syndrome (ARDS) / non-cardiogenic pulmonary oedema. This has been postulated by Lichtenstein and others to be due to an associated inflammatory exudate, which ‘glues’ the lung to the chest wall.
c. Other disease which ‘glue’ the lung to the chest wall, notably malignancy and previous pleuradhesis
d. Underventilated lung: for example, severe pain from rib fractures will ‘splint’ the chest. This can be problematic, as it is in precisely such patients with chest trauma that a pneumothorax must be excluded. Patients with severe chronic airways disease often demonstrate reduced lung sliding, particularly if bullae are present.
e. Unventilated lung: for example the left lung in a patient with right main bronchus intubation, or a lung in which the main bronchus is obstructed.
2. Is there pleural fluid?
Pleural fluid is best observed at the most dependent area of the thorax (see note above on image acquisition). Its appearance on LUS depends on its consistency:
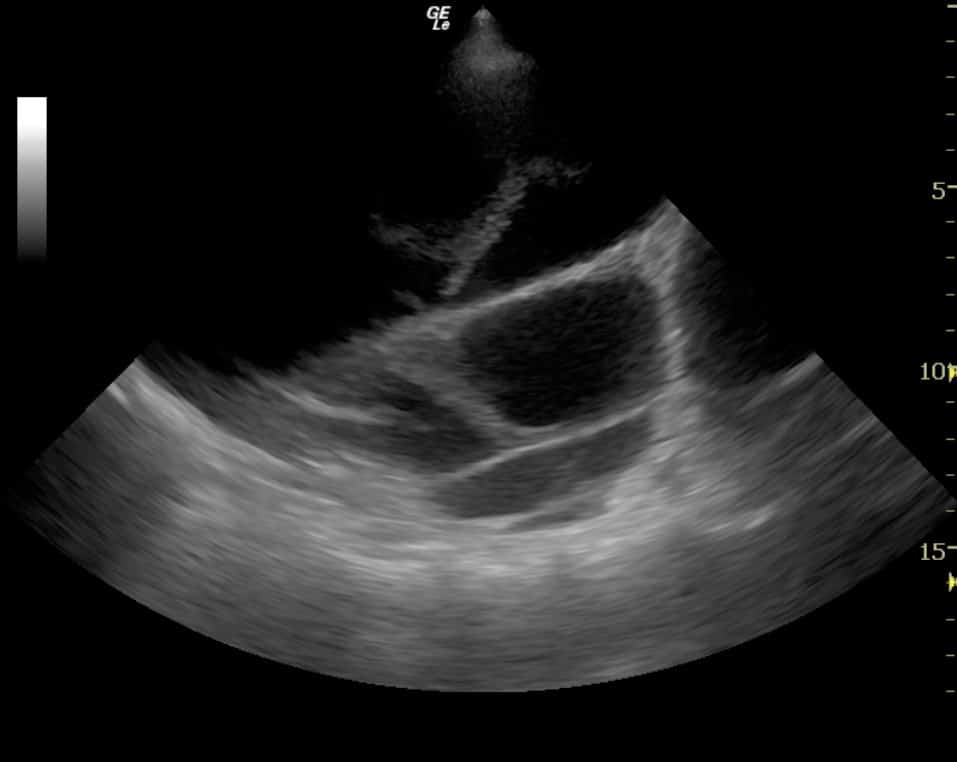
- Transudate or an acute haemothorax = ‘echo-poor’ (dark) (Figure 7)
- Complex collections (e.g. clotted blood or established exudates) = heterogeneous appearance (Figure 8)
The key features of pleural fluid on LUS are:
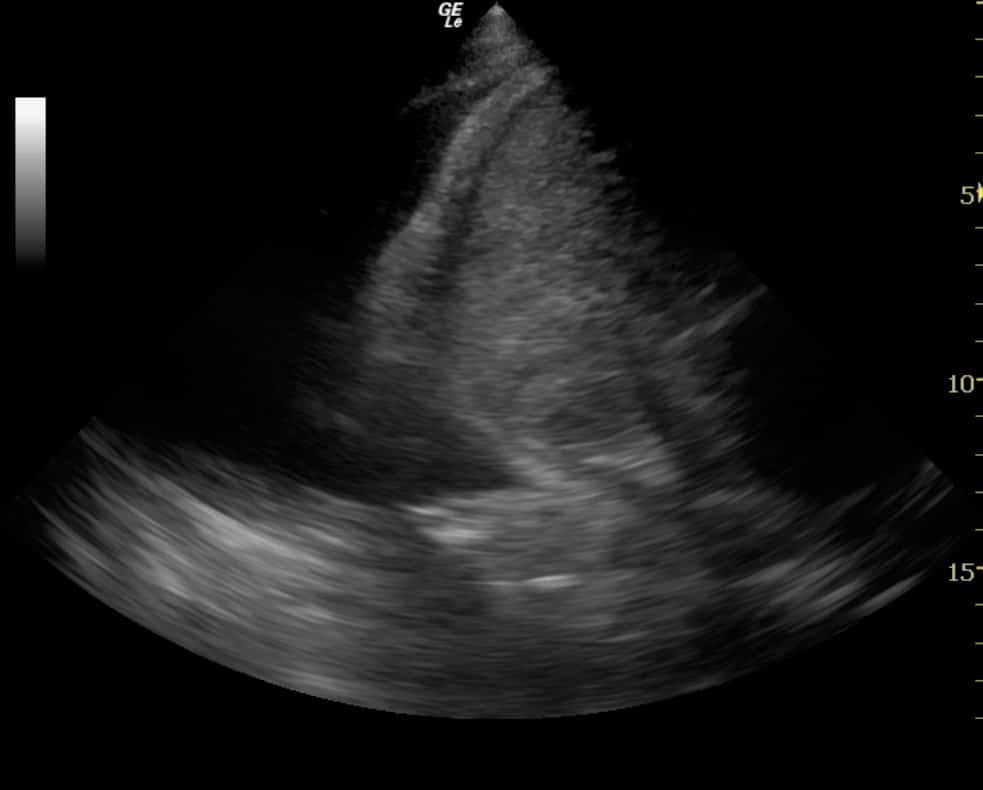
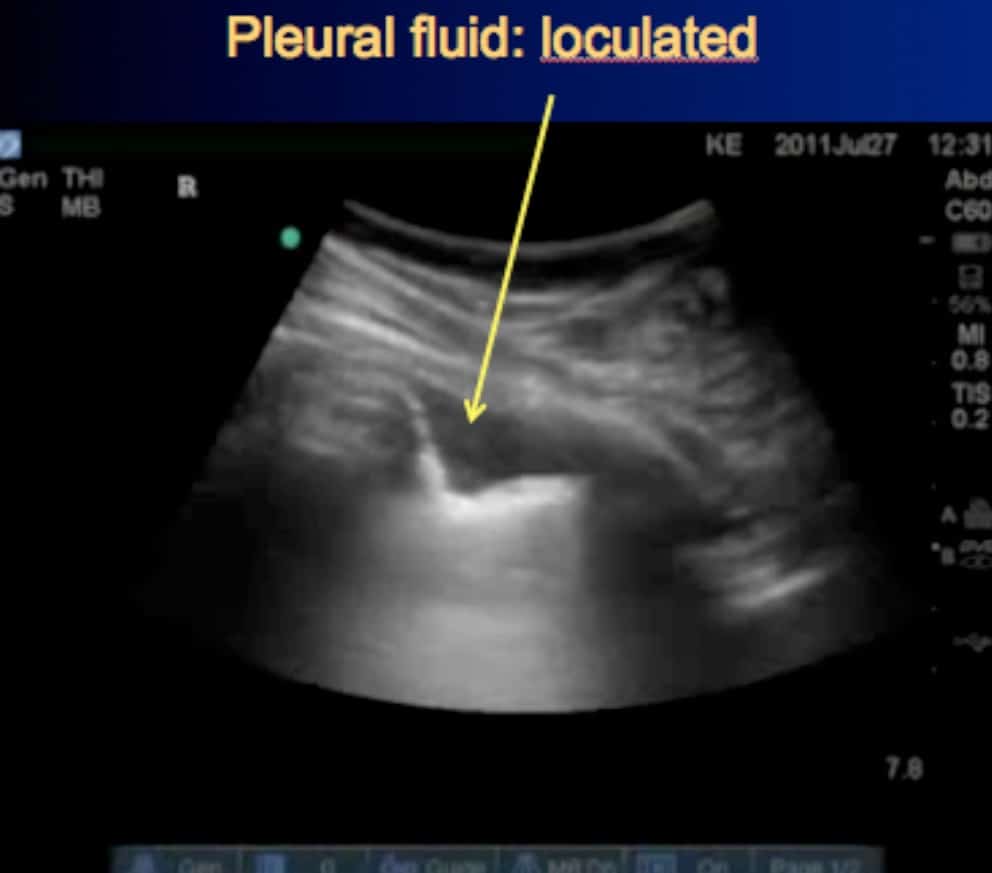
- Location: in the space between the parietal and visceral pleura, at the most dependent part of the thorax (unless large). If small, it may appear simply as a ‘stripe’ between the parietal and visceral pleura. If loculated, it may appear as a small ‘wedge’. (Figure 9) If large, it will appear as a large fluid collection in which the lung (and even the heart) may be seen to float.
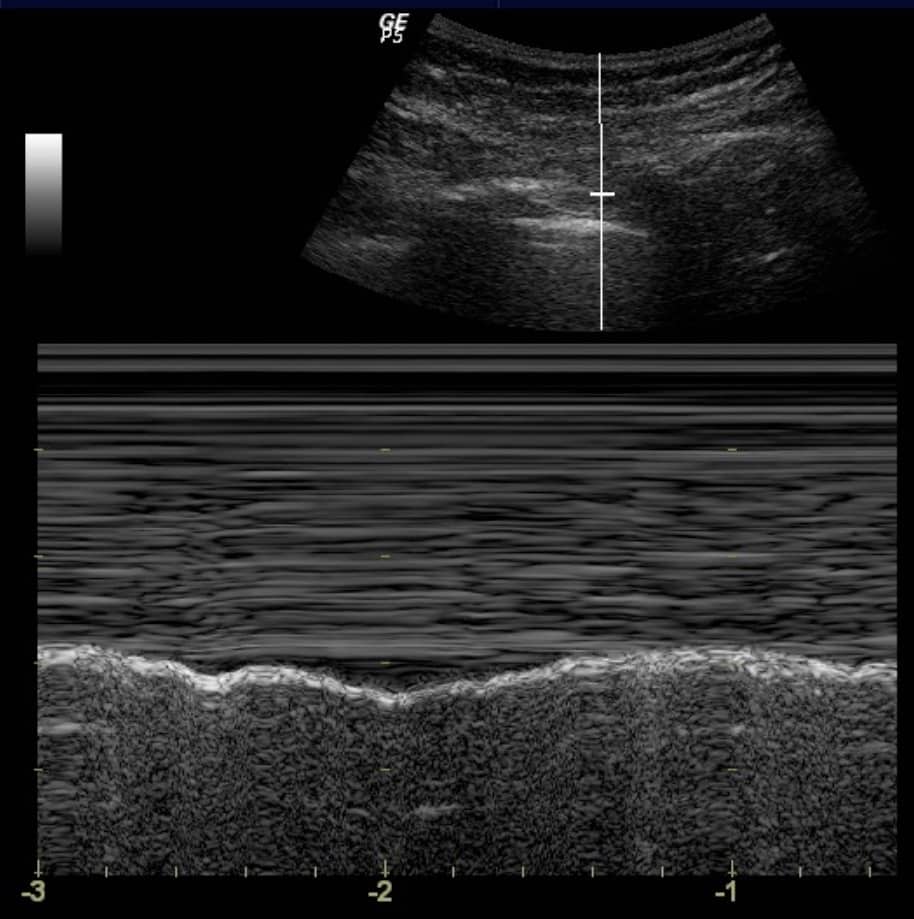
- Lung movement with respiration: unless the effusion has ‘glued’ the lung to the chest wall (see note above on absent lung sliding), the lung ‘bobs’ back and forth within the effusion with respiration. This is even visible when the effusion is small, and is sometimes rendered more obvious when using M-mode, in which case the movement resembles a wave (Lichtenstein’s sinusoid sign). (Figure 10)
3. Are there A lines, B lines or no lines at all?
- Artifacts are the basis of LUS
- The artifacts generated by a dry lung differ completely from those generated by a wet lung.
(a) A lines: Dry air (whether in a pneumothorax or healthy lung) is a powerful reflector of sound waves. So much of the sound wave is reflected at the pleural line that the wave ‘bounces back’ to the probe. But the surface of the probe is also a reflector, and some of the sound wave bounces back to the pleural line. If this happens again, multiple ‘echoes’ of the pleural line appear, evenly spaced and parallel to the pleural line. These lines are reverberation artifacts, and have been specifically termed A lines when they arise from the pleural line. (Fig 11.)
(b) No lines at all? If no lines are seen, this has the same significance as the presence of A lines: it implies dry air (either dry lungs or PTX).
(c) B lines: (Figure 12)
- Previously termed ‘comet tails’ (and ‘rockets’ when multiple)
- Bright (echogenic) artifacts that arise at the pleural line
- Move with respiration
- Efface other artifacts (such as A lines) as they move
- Persist to the bottom of the US screen without fading.
Multiple B lines (figs 13, 14) are found in two pathological conditions: pulmonary oedema and pulmonary fibrosis. They appear to be due to changes within the interstitium of the lung itself. For this reason, the LUS finding of multiple B lines in a lung field is termed ‘interstitial syndrome’.
Key points about B lines:
- Interstitial syndrome is a term you may have heard of in the literature. It is specific to LUS. It is not a clinical syndrome and in fact encompasses all the clinical syndromes that cause pulmonary fibrosis or pulmonary oedema. (The LUS differentiation of pulmonary fibrosis and oedema is addressed below.)
- B lines are never found in pneumothorax. They are only found when lung abuts the pleura. Therefore the presence of even a single B line rules out a pneumothorax in that intercostal space.
- Even a single B line rules out a pneumothorax at that site.
- The more B lines present, the ‘wetter’ or more fibrosed the lung (depending on the context). One or two B lines per intercostal space can be considered normal. Three or more are considered abnormal and are termed ‘B pattern’ (previously ‘rockets’).
- You need three or more B lines in an intercostal space before that bit of lung can be called ‘wet’.
- In the presence of confluent B lines, one can estimate the percentage of the intercostal space occupied by B lines and then divide by ten, for a rough estimation of the ‘number’ of B lines present. In figure 15, approximately 50% of the intercostal space on the right of the screen is taken up by confluent B lines, which equates to five B lines. However, in practice this is not usually required.
- However, even multiple B lines at the most dependent areas of the lung (usually near the diaphragm) have been observed in 25-33% of ‘normal’ individuals. I suspect this may be analogous to finding ‘incidental’ crepitations on auscultation of the lung bases of elderly patients.
- Up to 1/3 of normal subjects have multiple B lines in dependent regions.
Why are there B lines?
Although everyone agrees that B lines arise from thickened lung interstitium (interstitial oedema and fibrosis) no-one is really sure why. It has been postulated that the commingled air and fibre/fluid creates ultrasonic interfaces that cause ‘micro-reverberations’ that persist to the edge of the screen.
4. Is there consolidation?
- When the alveoli fill with fluid (blood, oedema) or solid material (pus, malignancy, pulmonary infarction) then they can conduct sound waves.
- If the areas of diseased alveolar tissue abut the pleura, these areas are no longer invisible on LUS, but become dark (sonolucent)
- Classically they appear as irregular, dark areas on LUS. (Figure 16)
- If very small (e.g. microconsolidation), they may appear simply as tiny irregularities along the pleural line, so that the pleura itself no longer appears smooth. (Figure 17)
Key points:
- Consolidated lung tissue appears dark (echo-poor) on LUS, but only if it abuts the pleural line. (If the consolidated area does not extend to the pleura, it will remain invisible on LUS.) Therefore LUS can be used to rule in pneumonia, but not to rule it out.
- The area in question may appear ‘liver-like’ (i.e. a similar echotexture), so it is crucial to ensure that the area in question is above the diaphragm This is all the more important because pneumonic consolidation often appears first at the portion of lung which abuts on the diaphragm.
- The deep margin of the area in question is irregular (figure 14), unlike the appearance of a loculated pleural effusion (figure 9).
- This appearance is common to the following diseases:
- Pneumonia, acute lung injury, atelectasis
- Malignancy, abscess (in which case fluid filled areas may also be present)
- Pulmonary infarction (e.g. pulmonary embolus)
- Certain LUS features may point to one disease rather than other, such as:
- Air bronchograms (pneumonia) (figure 18)
- Fluid filled areas (necrosis in malignancy, or abscess)
- Experienced scanners use Doppler imaging to differentiate some conditions based on their vascularity, for example:
- Pneumonia: normal vascularity preserved
- Malignancy: bizarre vascularity / areas of necrosis
- Infarct: flow only at edges
- However, that’s a little beyond the beginner.
For most clinicians, the best way to differentiate these conditions is on clinical grounds.
5. Is the pleural line irregular?
In the healthy lung and in some diseases (such as PTX and cardiogenic pulmonary oedema) the pleural line is smooth and regular. However, in some conditions it appears irregular, whether due to tiny microconsolidations which abut the pleura (pneumonia, acute lung injury) or due to thickening of the pleura itself (e.g. interstitial fibrosis, mesothelioma). (Figure 17)
Putting it together: the clinical questions
Many of the conditions described above share features on LUS, so it is sometimes difficult to differentiate them. Therefore it is important to bear in mind the following principles:
- Clinical context is paramount. We are clinicians first and sonologists second. For example if there is simply no way that the patient could have a pneumothorax, reevaluate the LUS findings and consider the differential diagnosis.
- Although the diseases in question may share individual features, the overall pattern of LUS findings is usually easy to differentiate. For example, both PTX and pneumonia may cause absent lung sliding, but pneumonia will be associated with one or more of the following: areas of consolidation, multiple B-lines (inflammatory oedema), irregular pleural line and pleural fluid. (Figure 24) None of these are found in PTX.
Clinical context is paramount. We are clinicians first and sonologists second
Pneumothorax versus pneumonia and other mimics
As noted above, many diseases can cause reduced or even absent lung sliding. Therefore this sign is simply not enough to ‘rule in’ PTX.
The classic features of pneumothorax (PTX) on LUS are:
- Absent lung sliding
- Absent B lines: even a single B line will rule out PTX. This will often be enough to tell PTX apart from its mimics. For example, as noted above pneumonia and acute lung injury often are associated with multiple B lines (inflammatory oedema).
- Presence of a ‘lung point’: unless the PTX has completely collapsed the lung, inevitably there will be an area where normal lung is still in contact with the pleura. Therefore it should still be possible to find an area of lung which still demonstrates normal lung sliding, and in fact it is often possible to demonstrate the transition point between PTX and normal lung on the same image: this is the lung point. (Figure 19)
The lung point sign has been described as 100% specific to PTX. However, in the author’s experience there are traps in over-reliance on the lung point:
a. Simple logic states that a complete PTX will not demonstrate the lung point, because the entire lung will be collapsed. Therefore, in precisely those patients in whom the clinician is most concerned about PTX (severe respiratory distress) there is little or no chance of finding a lung point.
b. A ‘false lung point’ can be clearly demonstrated on LUS in any patient in whom there are respiratory pauses. (Figure 18) this may cause real difficulty in the patient who is splinting his chest due to rib fractures (itself a high risk group for PTX). However, in this scenario every window will demonstrate exactly the same finding, whereas a true lung point will be found only along the margin between PTX and normal lung. Furthermore, on careful inspection the lung pulse should still be present in the areas of ‘stratosphere’ (see note 4 below).
c. Patients with areas of lung adherent to the pleura (e.g. malignancy) will demonstrate a convincing lung point on LUS. They may be extremely breathless due to their underlying disease or other causes such as pulmonary embolus. However, they will often demonstrate other LUS features that rule out PTX (e.g. B lines).
4. Absence of a ‘lung pulse’: this is the transmitted pulsation through lung tissue from the heartbeat. Usually not seen on B-mode (‘standard’ 2-dimensional) US, it is rendered more obvious in M-mode. (Figure 29) It is not present in PTX.
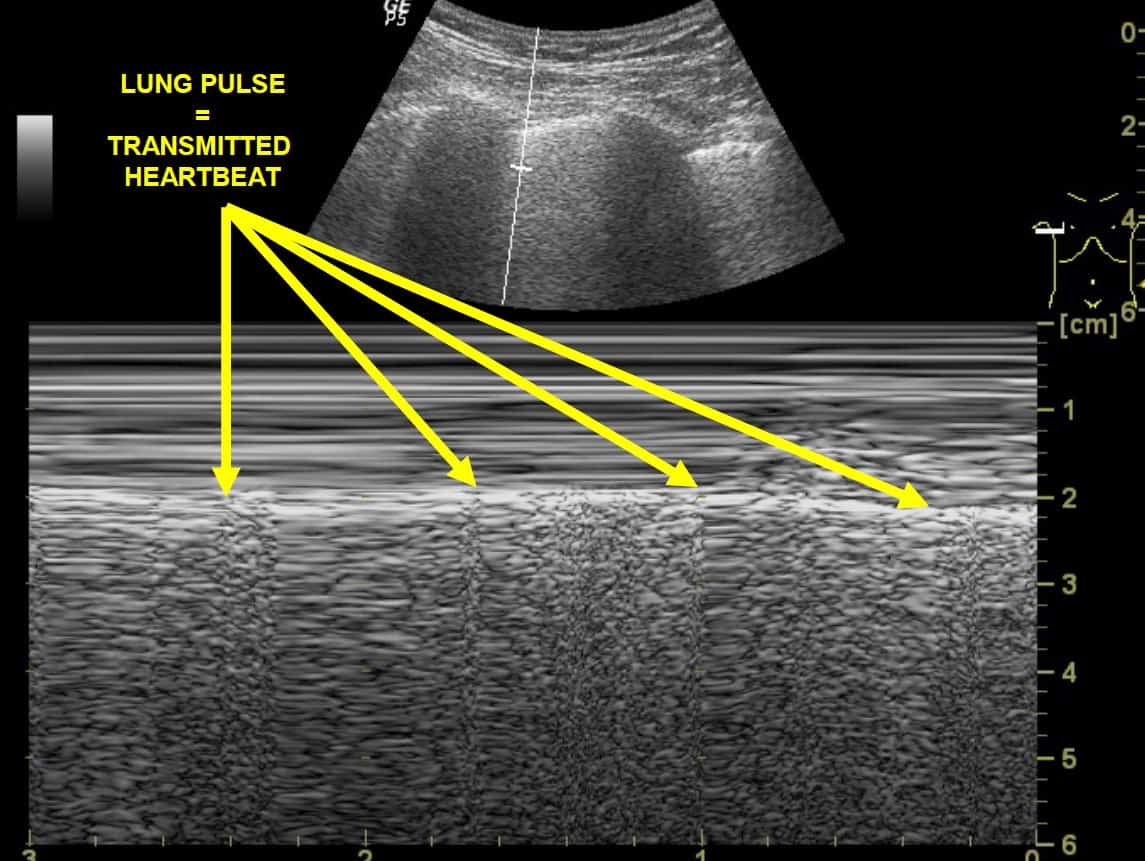
In the end, if one is simply unable to tell the difference between PTX and its mimics in a given patient, then one must rely on clinical judgement and act accordingly (for example urgently decompress the chest if it appears that a tension pneumothorax is probable).
Loculated pulmonary effusions versus small consolidations
These can appear similar, but a loculated pleural effusion will be smooth and well-defined (figure 9) and the lung may demonstrate ‘sinusoid’ movement beneath it on M-mode (figure 10). By contrast, a small area of consolidation will demonstrate an irregular lower margin and perhaps some of the other signs associated (such as comet tail artifacts), as noted above (figure 14).
Pneumonia and other causes of consolidation
As noted above it can be difficult to tell between causes of consolidation on LUS, for example multiple pulmonary infarcts from a shower of small pulmonary emboli can mimic multiple areas of pneumonic consolidation. If clinical assessment does not provide the answer, seek associated features of pneumonia such as air bronchograms (highly echogenic on LUS, figure 16).
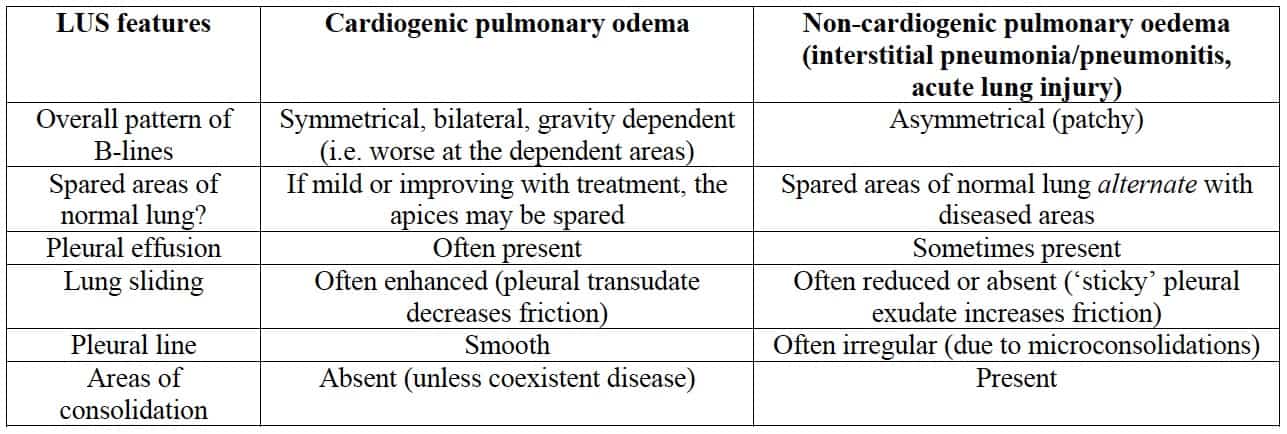
Cardiogenic pulmonary oedema versus widespread non-cardiogenic pulmonary oedema
(pneumonia, acute lung injury)
This can be a tricky one, both clinically and on LUS.
- Both demonstrate multiple B-lines.
- The key difference is that cardiogenic pulmonary oedema is hydrostatic while non-cardiogenic oedema is inflammatory.
The LUS differences are as follows:
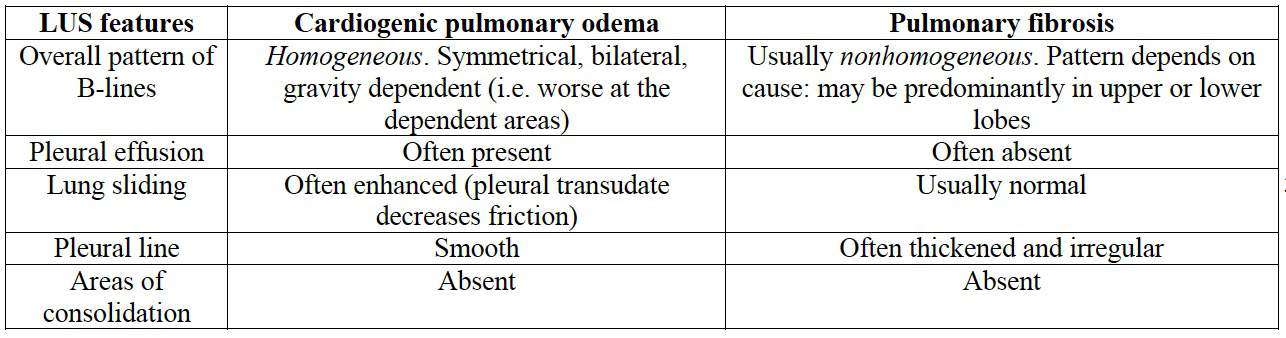
Cardiogenic pulmonary oedema versus pulmonary fibrosis
Similarly, these conditions can be difficult to differentiate clinically (e.g. both may cause right heart failure) and worse, can coexist. The LUS differences are as follows:
Causes of localised B-pattern
Multiple B-lines (B pattern) can arise from any cause of pulmonary oedema, such as inflammation or trauma. Hence, if LUS demonstrates a localised area of B-pattern (multiple B lines) in an otherwise normal lung, consider the following differential diagnoses:
- Normal subject (if present only at the most dependent areas)
- Localised pneumonia / pneumonitis
- Pulmonary contusion
- Pulmonary infarction
Matching the LUS findings with the disease
Normal lungs:
- Dry(-ish) air
- Scatter, often with A-lines
- Up to 2 B lines per window are OK
- No chunkiness
- No pleural fluid
Pneumothorax:
- Very dry air
- Usually see A-lines
- No sliding
- No B lines
- No lung pulse
- See a lung point unless lung is completely collapsed
APO:
- B profile = Plenty of B lines in all windows
- Lung sliding preserved
- Often see effusions
ARDS or pneumonia:
- Lungs might look wet
- lung rockets in all windows
- lung sliding reduced / absent
- Pleural line may be irregular
- Lungs might look patchy (wet / dry areas)
- Lungs might look chunky
Pulmonary Embolus:
- Lungs usually look dry.
- Sometimes you see chunks.
Asthma/ COPD:
Lungs usually look dry
Traps to avoid in LUS
Pneumothorax
- False seashore sign: in the presence of chest wall movement (e.g. marked respiratory distress) or probe movement, the entire LUS window may move, creating a ‘false’ seashore sign. Therefore only accept a ‘seashore sign’ when it is clear that there is only movement below the pleural line.
- False lung point sign and over reliance on the lung point sign: see note above.
- Colour and Power Doppler are sometimes recommended to render lung sliding more obvious.
- However, in the author’s experience there is simply too much risk of a false positive finding with movement of the chest wall and of the probe itself, particularly for novice scanners.
- False seashore sign due to scanning the heart instead of the lung: when scanning the left chest, one may mistake the heart’s movement artefact for lung movement (figure 27). A moment’s reflection should suffice to remind the operator that normal lung sliding is horizontal with respect to the probe (figure 9), unlike the heart’s movement which has a vertical component.
- Missing a small PTX: this hazard is unlikely if one scans the highest part of the lungs. However, it is still possible to miss a small ‘loculated’ PTX with this approach, so if it is considered essential to completely rule out a PTX, one should scan as much of the lung as is possible. (Note that this is rarely clinically indicated.)
Consolidation
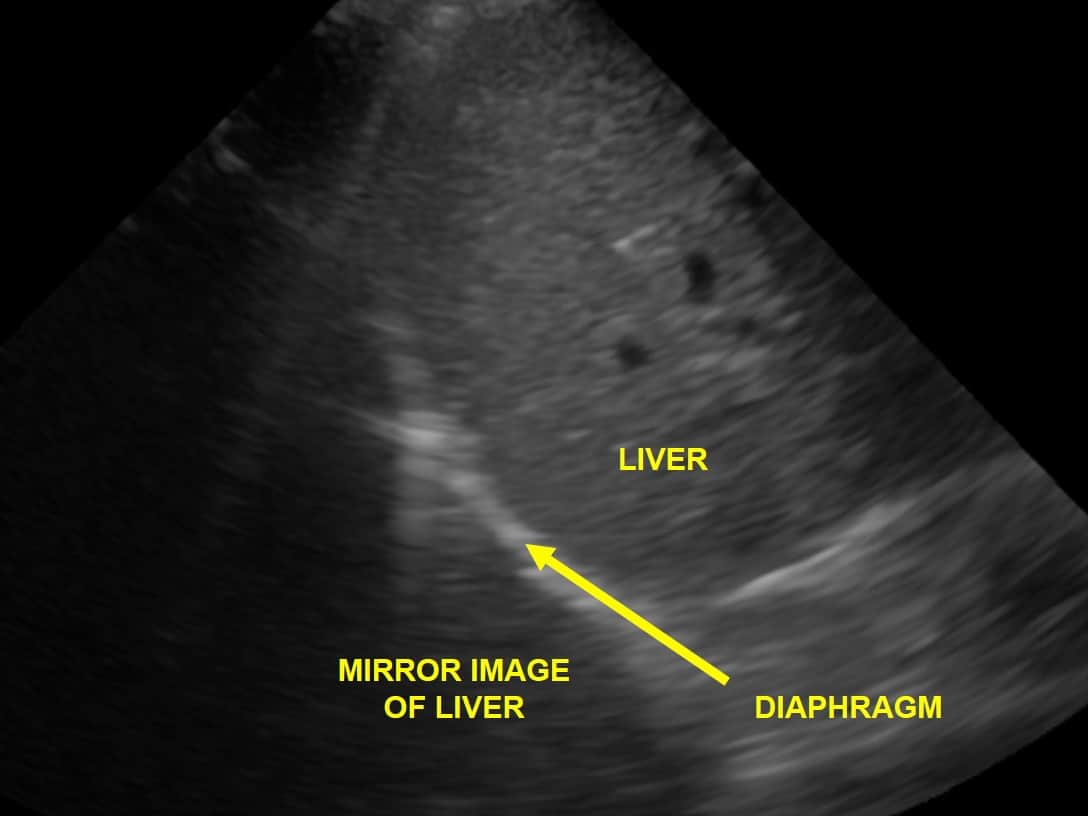
- Mirror image: at the base of the lungs (i.e. just above the diaphragm), make sure that any apparent ‘consolidation’ is not actually mirror artifact. (Fig 23)
Appendix A. Lichtenstein’s BLUE and PLAPS points
With the aim of standardising lung scanning, Lichtenstein defined three points to place one’s probe:
- The upper BLUE point: the root of the middle & ring fingers of a hand (of the same size as the patient’s) placed just below the clavicle, fingertips on the midline of the sternum.
- The lower BLUE point: the middle of the palm of a 2nd hand placed just below the first.
- The PLAPS point: the posterior continuation of the lower BLUE point (as far around as you can get the probe).